One of the most important factors in wine is the concentration of hydrogen ions. The physico-chemical characteristics of wine, sensory factors and even micro-biological stability depend on the number of these hydrogen ions. The concentration of these ions is measured in logarithmic concentration and is called pH for short. pH = -log [H +] pH values are divided in the range 0 to 14, where there are 7 neutral liquids. 0-7 are acidic liquids and 7-14 are alkaline liquids. It should be noted again that these data are compiled on a logarithmic scale, which means that for example the pH value between 3 and 4, as a result of the logarithmic dependence, pH-3 is 10 times stronger than pH4. pH7 has pure water. The pH of wine is mainly between 3 and 4. Wine contains mainly weak organic acids, namely tartaric acid, lactic acid, citric acid, malic acid and acetic acid. Weak acids, in comparison with strong acids, are characterized by a degree of weak dissociation, hence the concentration of hydrogen ions, in this case, is relatively low. Degree of dissociation pK - is the dissociation constant, which has different meanings for different acids. In weak acids (in the case of acids in wine) this ratio is generally very small (0.0001, or less), therefore the dissociation of these acids in wine is about 1%. Hydrogen ion concentration vs. Titral and total acidity. Often many people make the mistake of associating pH with total acidity. In fact, there is a very significant difference between the two. As mentioned above, pH is the concentration of hydrogen ions (protons). Total acidity, as the name suggests, is the concentration of acids in a liquid. Their measurement techniques are also different. pH is measured with a fairly simple instrument called a pH meter. This tool costs about $ 20 (higher precision devices are more expensive) and is a very important measuring tool for winemakers. To determine the total acidity, the so-called titration method is used. Total acidity is a very important parameter for getting quality wine. The acidity of the wine mainly ranges from 0.4-0.85% (4-7.5 g / l for wine acid), although in high quality wines the acidity is better in the range of 5.5-7.5 g / l. Wines with less acidity than this value do not have a great aging potential, are less protected from microbiological problems and are also relatively "sluggish" in organoleptic terms. Wines with excessively high acidity are microbiologically more stable, although they become uncomfortable to drink, especially if their balance is disturbed.
And yet why do we need so much information and analysis related to wine?
First of all, the need to do these analyzes is still in the grape juice to determine the degree of ripeness of the grapes, as well as the potential quality of the final product - wine, how balanced it will be. In addition, by measuring the pH we understand the degree of microbiological stability of the juice in the process of fermentation and then the wine. It depends on the pH how much sulfur dioxide will need to be added. To make the wine microbiologically stable. Often winemakers ignore these parameters and orally, on the advice of friends or relatives, start "sulfur" (potassium metabisulfite) in the wine to stabilize the wine. This is a bad practice because, as mentioned, the amount of sulfur dioxide needed to be added to the microbiological stabilization of the wine depends on the pH of the wine. This characteristic is different in all wines, therefore some wines may not need to add sulfur at all, while others may need to add more, and this amount may not perform its function. Therefore, after measuring the pH value, the winemaker will be able to determine the degree of stability of the wine. They need to find the required amount of sulfur dioxide in spreadsheets or online calculators, which are readily available on the Internet.
pH is measured with a special, small device called a pH meter. These devices come in a variety of prices and quality, even available within $ 15 on Amazon. Such, relatively inexpensive devices do not interfere with great accuracy. However, it is better not to have this cheap device. When purchasing this device, the main factor to consider is that it has an accuracy of 0.01, i.e. to measure 2 units after an integer. Most of these types of devices also have a calibration button. Also, instructions will follow - how to calibrate them.
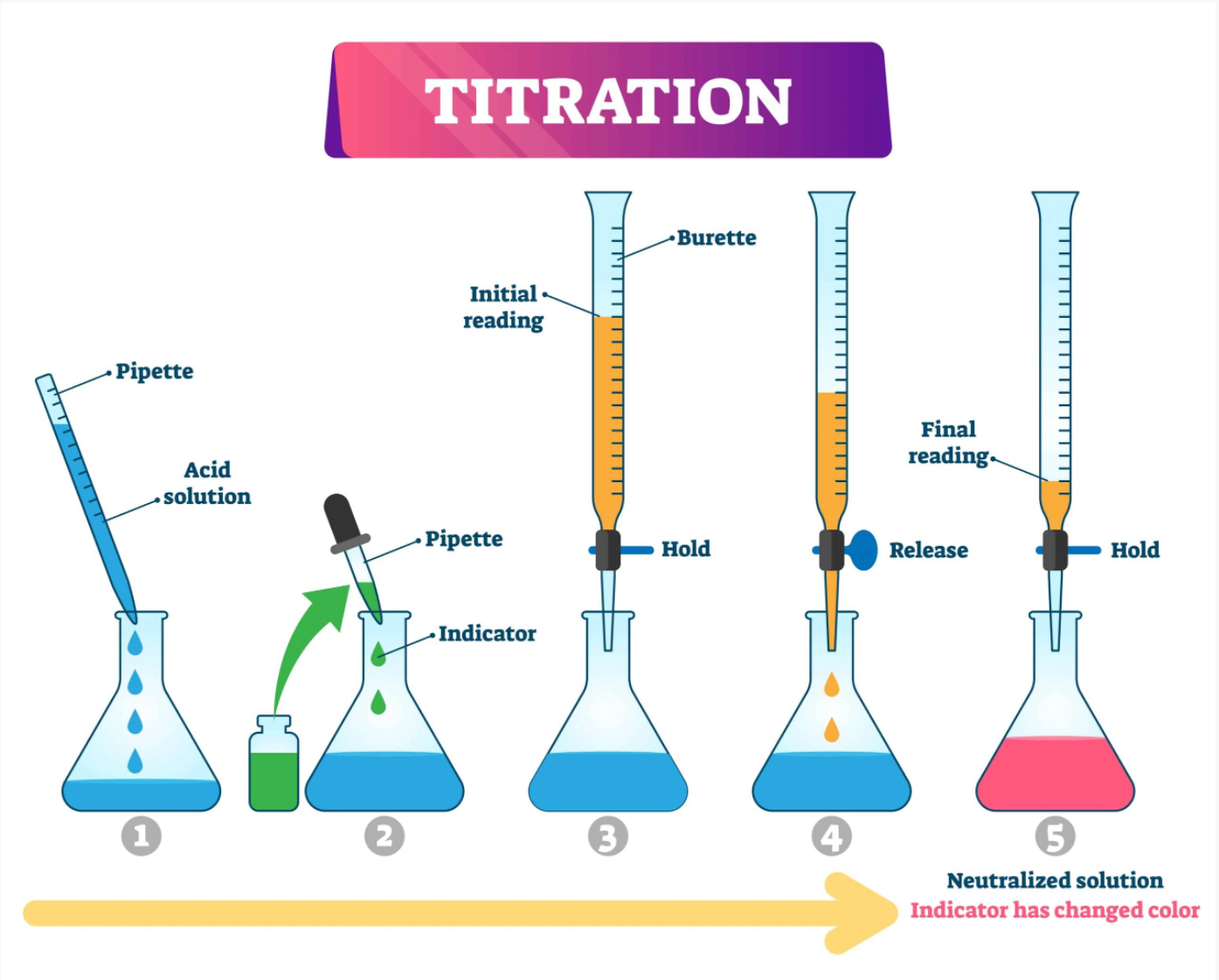
Calibration is necessary to periodically obtain a more or less accurate value. Measurement is very simple. Place the test liquid (grape juice or wine) in a container, immerse the measuring device in this liquid so that the liquid covers the glass electrode. Once placed in it, the pH output on the digital display will start to change (increase or decrease). The final value will be after stabilization (this can sometimes take 2-3 minutes, although in most cases it lasts an average of 1 minute). As for the overall acidity measurement, a little more work needs to be done here. For this, first of all, we will need the following reagents and equipment: Partitioned pipette or barrel Sinjara 1% phenolphthalein alcohol solution 0.1N normalized NaOH Glass jar to drain the liquid A magnetic stirrer is preferred, though not necessary. Procedure: Place the test liquid (grape juice or wine) in a clear glass jar or other container. Add 2-3 drops of phenophthalein solution, mix well and start titrating with sodium alkali solution until the test solution changes color. Titration occurs very slowly, with the addition of one drop at a time, with constant vortexing. After adding a certain amount of saturation solution, the color of the test liquid begins to change. When the liquid changes color, do not add more saturated solution. Wait 10-15 seconds, if the color is retained and does not return to the original color, then the titration is complete. If the color has returned to the original color, then we continue the titration again by adding drops. When we see that the color has stabilized, we note the amount of saturating fluid consumed and enter these data into the following formula to determine the total acidity: Total acidity (TA g / l) = (amount of solution consumed (NaOH) per ml) * 7.5 / (volume of wine sample in ml). For example, if we take 10 ml of wine for testing, and 8 ml of NaOH was used for testing, and we enter this data into the formula, then we get: TA (g / l) = 8 * 7.5 / 10 = 6 g / l.